Our Science
NEX-I, First-In-Class Cancer Immunotherapy Development Company
Core Technology


NEX-I aims at developing first-in-class drugs overcoming tumor resistance to the current immunotherapeutics. Over 20 years, the scientists at NEX-I have established a profound immuno-oncology platform to address resistance mechanisms against the current anticancer therapies with their causing factors. ONCOKINE®, a group of protein derivatives secreted in the cancer refractory environment, was first addressed by NEX-I for alternative treatment options. NEX-I has extensively examined tumor tissues and blood samples of cancer patients treated with various immunotherapeutics for clinical relevance. NEX-I developed standardized animal models to validate the targets and confirm the efficacy of tumor growth suppression. NEX-I employs a unique and innovative platform to predict human response most accurately by analyzing complex human patient-derived models containing organotypic spheroids and humanized mice systems.
ONCOKINE®
ONCOKINE® is a group of protein derivatives primarily present in the tumor microenvironment. These factors are the leading causes of immunotherapy-resistance of cancers. NEX-I herewith ambitiously develops innovative therapeutics targeting ONCOKINE® to cure the immunotherapy resistance of cancers.


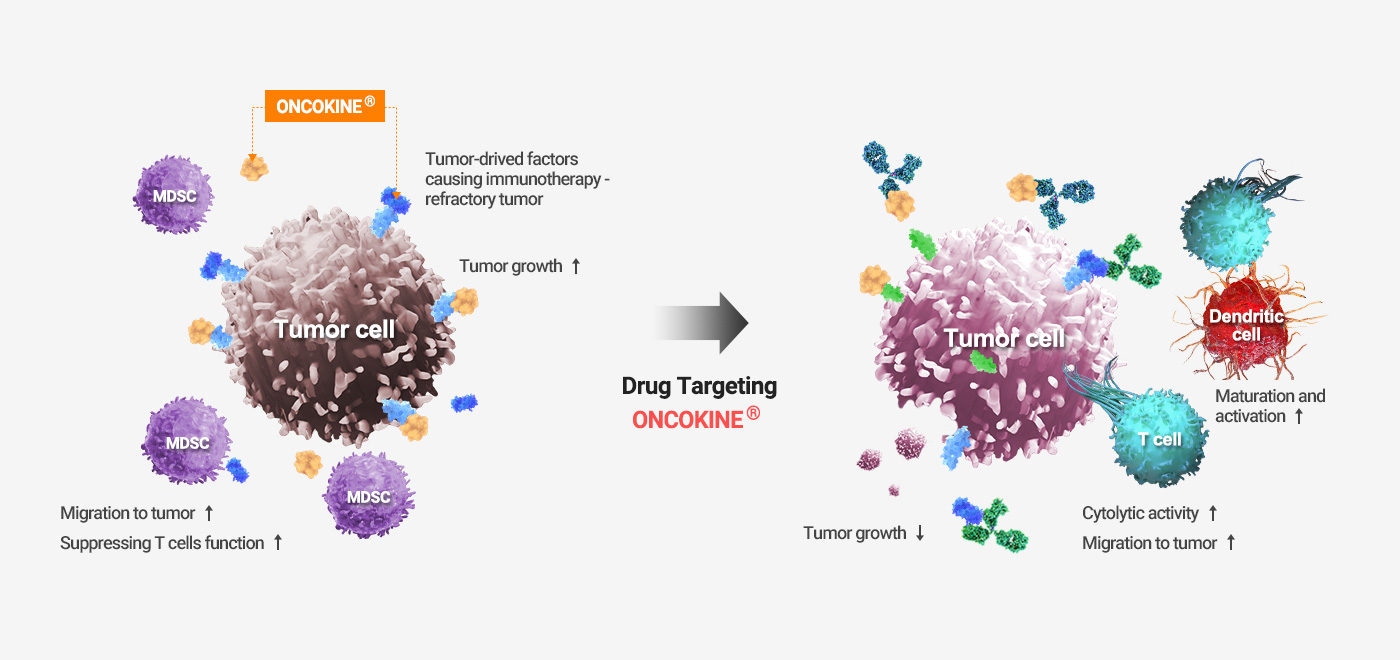
Tumor-derived factors that mediate the intrinsic and extrinsic resistance against immunotherapy
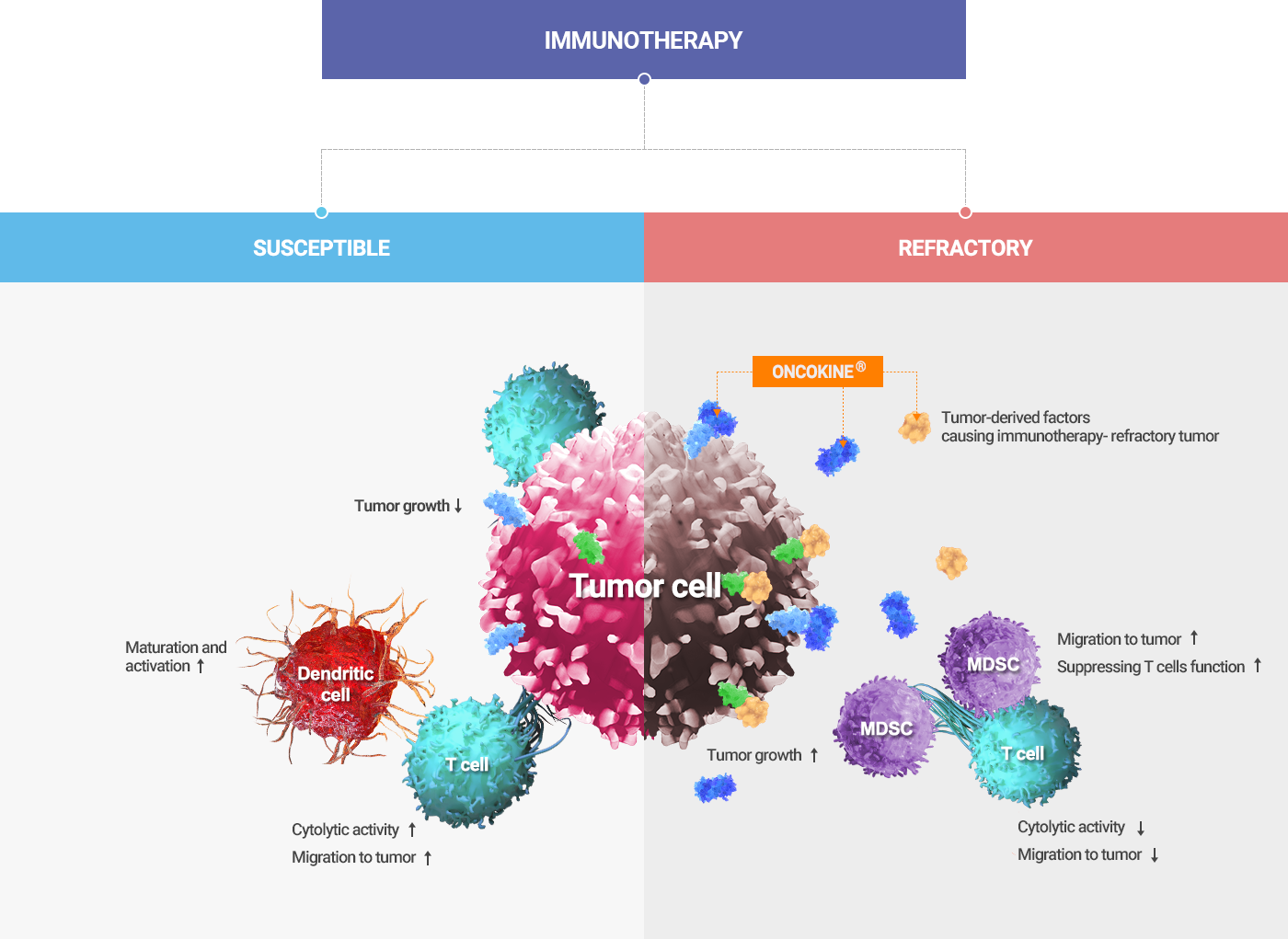
- When cancers respond to immunotherapies, the immune system is expected to control tumor growth by activating dendritic and cytolytic T cells.
- In immunotherapy-resistant cancers, ONCOKINE® is often present in the microenvironment and causes immune evasion of the cancer cells.
ONCOKINE® mediates the intrinsic and extrinsic resistance against immunotherapies. ONCOKINE® functions not only to promote cancer cell growth but also to recruit immune suppressive cells such as MDSC and tumor-associated macrophages (TAM), and by doing so, it dominates the immune system by restraining cytolytic activities and infiltration of cytolytic T cells and natural killer (NK) cells.
Target Discovery
Establishment of immunotherapy-refractory tumor models
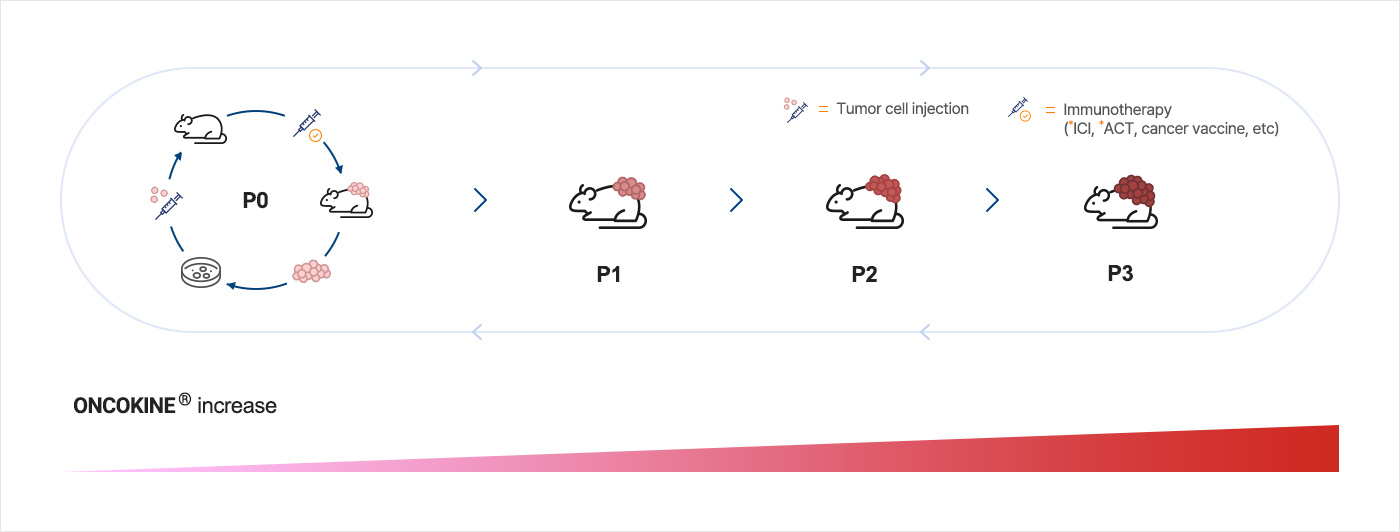
* ICI : Immune Checkpoint Inhibitor / * ACT : Adoptive Cell Transfer
NEX-I has established immunotherapy-refractory cancer models to analyze its characteristics and discover new drug targets for potential treatment. Through in vivo animal studies applied with various types of immunotherapies such as immune checkpoint inhibitors (anti-PD-1 or anti-PD-L1 antibody) treatment, antigen-specific T cell transfer technique, and cancer vaccine, etc., attained immunotherapy-refractory cancer cell P3 from the general P0 Confirmed increases of ONCOKINE® as the cancers progress with the immunotherapy-refractory environment. We have developed over 20 immunotherapy-refractory cancer models, including non-small cell lung, colorectal, melanoma, liver, cervical, etc. We validate targets through various proof of concept translational research, animal via syngeneic tumor models and multiple human patient-derived models.
Biomarker and Clinical relevance
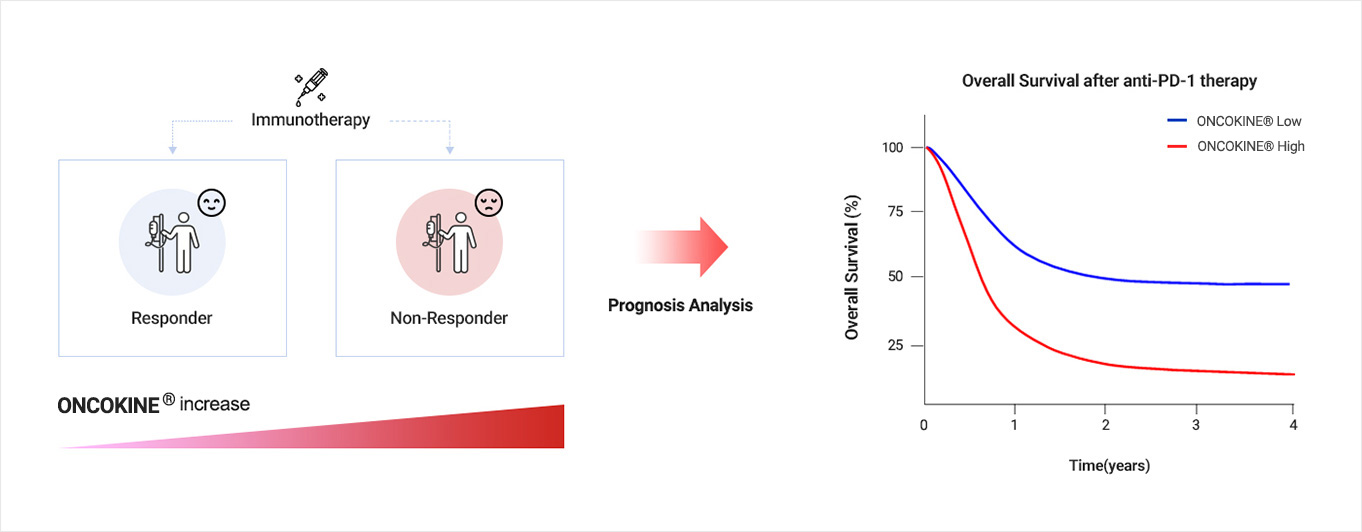
In addition to the animal models, NEX-I has robustly analyzed tumor tissues and blood samples of cancer patients for clinical relevance. ONCOKINE® is highly expressed in tumors and blood of non-responding patients against ICI therapy, and its progressive ability to induce immunotherapy-resistance to the current treatment was also confirmed. Patients with low levels of ONCOKINE® had significantly longer overall survival than those with the high expression. Studies revealed a positive correlation between ONCOKINE® levels in tumor tissues and the anticancer effect of anti-ONCOKINE® drugs.
Drug Targeting
NEX-I is developing first-in-class cancer immunotherapeutics that target ONCOKINE®.